
| Categories |
|---|
| Mechanics |
| Fluid Mechanics |
| Oscillation and Waves |
| Thermodynamics |
| Electricity and Magnetism |
| Optics |
| Modern Physics |
| Astronomy |
| Images |
|---|
 |
 |
 |
[Setup Time: 15 Min.] [Current Condition: Good]
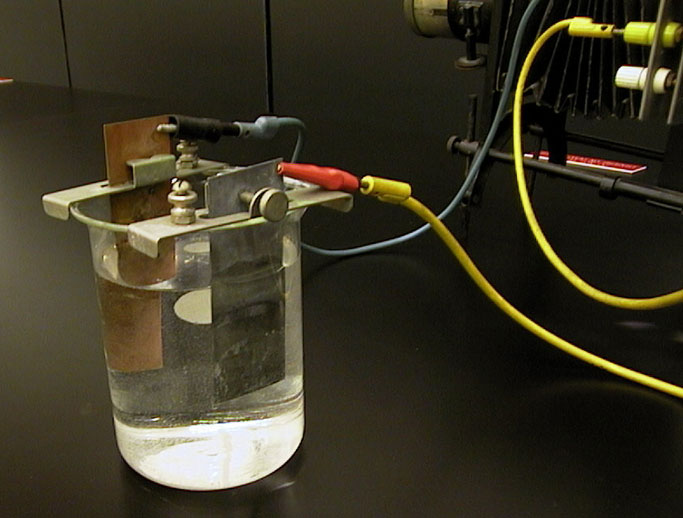
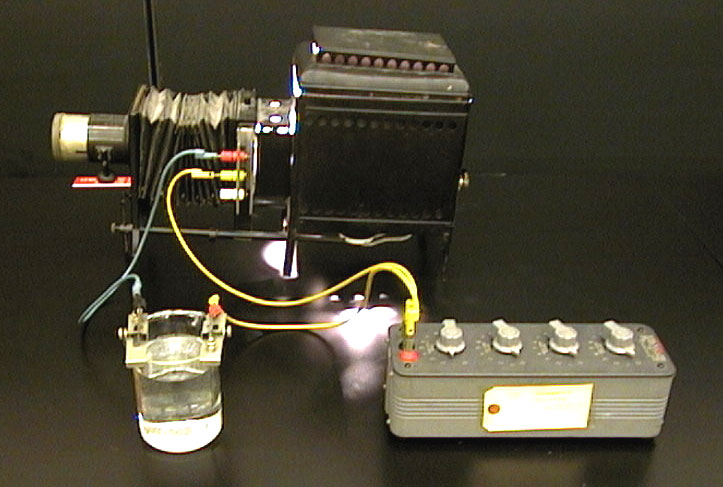
Copper and zinc electrodes are placed in a diluted hydrochloric acid solution, and the resulting current is displayed on a galvanometer projected onto the screen. The separation between the electrodes can be varied, and the resulting change in current observed.
1. Pour a drop of HCL solution from the flask into the beaker filled with water (using gloves).
2. Hang electrodes (one zinc and one copper) on the sides of the beaker and attach leads to the electrodes with alligator clips.
3. Connect other end of the leads to the projection meter and slide it into projector.
4. Place the projector roughly in the center of the front bench and point it at the screen.
Note: Brown bottle has a roughly 10% HCL solution which can be diluted with water when solution in flask is used up.
HCL solution in tall flask in Chemical Cabinet
Beaker in G. E&M, 1B
Wire Leads in L. Misc
Box of Electrodes in G. E&M, 1C
Projection Galvanometer in F. E&M, 1C
Overhead in L.Misc
N/A
N/A
N/A